Drug Pipeline
Having completed pre-clinical & formulation work with the University of Toronto, we are advancing to late-stage clinic development in Clinical High Risk for Psychosis, as well as Phase II trials elsewhere within the CNS space.
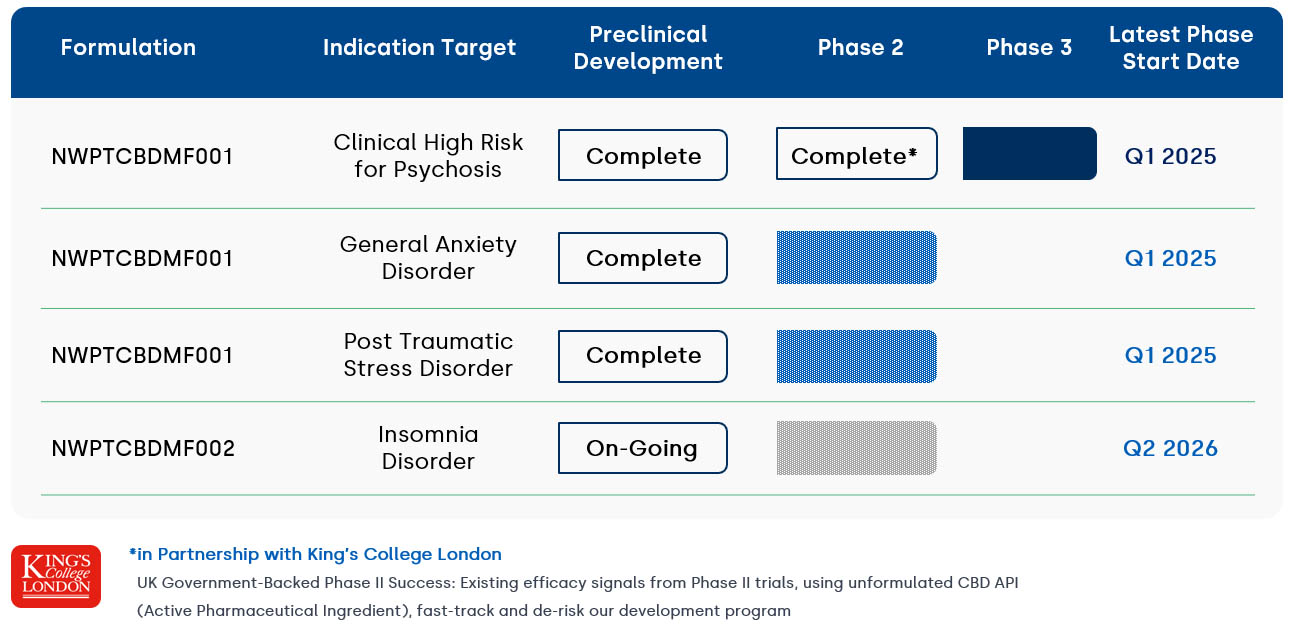
Having completed pre-clinical & formulation work with the University of Toronto, we are advancing to late-stage clinic development in Clinical High Risk for Psychosis, as well as Phase II trials elsewhere within the CNS space.
